
ISO 9626
Stainless Steel Needle Tubing Test
ISO 9626 is the international standard that defines the mechanical properties and dimensional requirements for rigid stainless steel needle tubing used in medical devices, including syringes and hypodermic needles. As syringe needle testing plays a critical role in ensuring safety and functionality, understanding and applying ISO 9626 helps manufacturers meet regulatory requirements while delivering reliable medical components.
What is ISO 9626?
It provides standardized specifications for stainless steel tubing used in the manufacture of medical needles, ranging from 3.4 mm (10 Gauge) down to 0.18 mm (34 Gauge). The standard applies only to rigid tubing, excluding flexible variants due to differing mechanical behaviors.
Key areas covered by ISO 9626 include:
- Dimensional tolerances of stainless steel needle tubing
- Mechanical properties such as stiffness and resistance to breakage
- Corrosion resistance under simulated physiological conditions
- Preparation and evaluation of extracts for biocompatibility testing
The standard is essential for manufacturers producing needles for injection, fluid sampling, and other medical applications. Adherence ensures performance consistency, patient safety, and compliance with global regulatory bodies.
Syringe Needle Testing per ISO 9626
ISO 9626 outlines several crucial syringe needle testing procedures that validate the mechanical integrity and durability of needle tubing:
Injection Needle Stiffness Test
This test evaluates the rigidity of the needle tubing under a controlled load. The procedure involves:
- Supporting the tube on a span determined by its gauge
- Applying a downward force via a plunger with a specified geometry
- Measuring deflection at the center to assess stiffness
Excessive flexibility may result in poor handling or inaccurate injections. Manufacturers must ensure that the needle maintains mechanical stability under expected clinical use conditions.
Cell Instruments’ TS-01 Needle Tubing Stiffness Tester is optimized for this application. It offers:
- ±0.1 N force accuracy
- Micron-level deflection measurement
- Customizable span settings per gauge specification
Needle Break Resistance Test
Break resistance is vital to prevent tubing failure during use. ISO 9626 prescribes a cyclic bending test, where tubing undergoes up to 20 alternating bends at a defined angle:
- Regular-walled tubes: (25 ±1)°
- Thin-walled tubes: (20 ±1)°
- Extra-thin/ultra-thin-walled: (15 ±1)°
The test helps assess fatigue resistance under repeated stress. A visible fracture indicates failure.
Cell Instruments’ NTT-01 Needle Tubing Break Resistance Tester performs this test with:
- Programmable bending angles and cycles
- Visual inspection fixtures
- Adjustable clamping for various tube diameters
Corrosion Resistance Evaluation
Annex D of ISO 9626 addresses corrosion resistance, ensuring needles do not degrade in contact with body fluids. Stainless steel tubes must resist pitting and rust formation after immersion in test solutions like saline.
Although not always mandatory, many manufacturers include corrosion resistance as part of routine syringe needle testing for risk mitigation.
Additional ISO 9626 Testing Considerations
Apart from the core mechanical evaluations, ISO 9626 also requires:
- Precise dimensional measurements
- Identification of wall type (RW, TW, ETW, UTW)
- Alloy classification per ISO 15510 or ASTM A959
- Test documentation including gauge size, deflection values, and test conditions
Accurate testing not only satisfies compliance requirements but also strengthens product reliability and brand reputation.
ISO 9626 Testing - Essential for Syringe Quality Control
Failure to meet ISO 9626 standards can lead to:
- Needle bending or breakage during injection
- Inconsistent fluid delivery
- Increased patient injury risks
- Regulatory non-compliance and recalls
By integrating ISO 9626-compliant syringe needle testing, manufacturers and quality assurance teams:
- Validate mechanical strength and durability
- Ensure compatibility with regulatory requirements
- Reduce liability and product failure rates
- Improve customer trust and satisfaction
Choosing ISO 9626 Testing Solution

TS-01 Syringe Needle Stiffness Tester
To accurately assess the stiffness characteristics of stainless steel needle tubing as specified in ISO 9626 Annex B, the TS-01 Syringe Needle Stiffness Tester from Cell Instruments provides a reliable and high-precision solution. This instrument ensures consistent application of force and accurate measurement of tubing deflection, helping manufacturers and quality control teams meet regulatory expectations for injection needle stiffness testing.
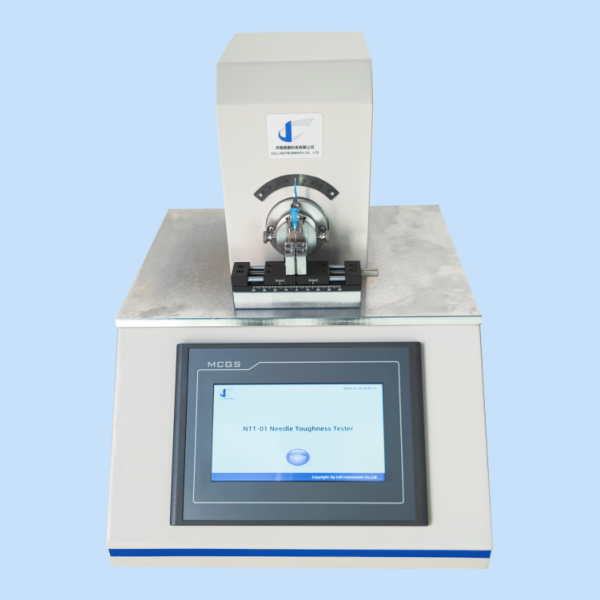
NTT-01 Needle Break Resistance Tester
For evaluating the mechanical durability of medical needle tubing under repeated bending forces per ISO 9626 Annex C, the NTT-01 Needle Break Resistance Tester is an essential tool. Designed for rigorous quality assurance environments, this tester simulates real-use conditions and delivers precise data on tubing resilience and structural integrity—critical for preventing needle failure during clinical application.
Contact Us Get ISO 9626 Testing Solution
ISO 9626 plays a pivotal role in syringe needle testing by setting benchmarks for stiffness, break resistance, and overall structural integrity of stainless steel needle tubing. Through precise mechanical evaluations, manufacturers can ensure their products meet both regulatory and functional expectations.
Cell Instruments’ TS-01 and NTT-01 testers offer a comprehensive, ISO-aligned solution to meet the evolving demands of medical device quality control. Invest in better compliance, reduced risk, and superior performance with Cell Instruments as your partner in syringe needle testing.
FAQs
ISO 9626 defines the dimensional and mechanical requirements for rigid stainless steel needle tubing used in medical devices to ensure safety and performance.
Tests include injection needle stiffness, needle break resistance, corrosion resistance, and extract preparation for biocompatibility evaluation.
These refer to wall thickness categories: Regular Wall (RW), Thin Wall (TW), Extra-Thin Wall (ETW), and Ultra-Thin Wall (UTW), which determine test parameters and bending angles.
Cell Instruments’ TS-01 and NTT-01 provide accurate, repeatable test conditions for stiffness and break resistance, helping manufacturers comply with ISO 9626 standards.
It ensures the needle won’t fracture under stress during use, preventing device failure and patient injury.
